Colloids are around us everywhere. Colloidal chemistry is also an essential part of the chapter surface chemistry. To understand more about colloids, types of colloids and also their reactions, read on.
Wherever you turn you can see a colloid. The paint on the walls is a colloid. Shaving gel is a colloid. Butter is also a colloid! But what do we know about colloidal chemistry? Let’s start with the basics.
What is a colloid?
Colloids are neither elements nor states of matter. It is one of the primary types of mixtures. The other 2 types are suspensions and true solutions. The particle size in colloids is about 1 to 1000 nm. Colloid particles can pass through filter paper. This is also the middle ground between a true solution and a suspension.
Colloidal mixtures also appear translucent or unclear, diffuse slowly, and do not settle. This type of mixture is heterogenous.
Types of colloids
Colloids are formed by the mixing of a dispersed phase (discontinuous) and a dispersion medium (continuous). The continuous medium is the one present in a larger amount. Both can be in any state. The table below classifies colloids based on the permutations of discontinuous phases.
|
Dispersed phase |
Dispersion medium |
Colloid name |
Examples |
|
Gas |
Solid |
Solid foam |
Pumice |
|
Gas |
Liquid |
Foam |
Whipped cream |
|
Liquid |
Solid |
Gel/solid emulsion |
Cheese |
|
Liquid |
Liquid |
Emulsion |
Milk |
|
Liquid |
Gas |
Aerosol |
Fog |
|
Solid |
Solid |
Solid sol |
Gemstones |
|
Solid |
Liquid |
Sol |
Paints |
|
Solid |
Gas |
Aerosol |
Smoke |
Tyndall Effect
If you are ever confused about whether something is a colloid, there is an easy experiment you can perform to help yourself. Pour the mixture into a glass trough and shine a bright light through it. If the mixture is a true solution, the light passes completely without any visible beam of light showing in the trough.
If you can see a beam of light, then the mixture is a colloid. This is because colloidal particles cause light to scatter in different directions due to their size. This enables us to see the beam of light, unlike in the case of true solutions with smaller particles. Here is a picture illustrating the Tyndall Effect:
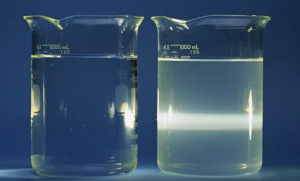
The beaker on the right contains a colloid because we can see a beam of light.
Classification based on interacting phases
We can also classify colloids based on how the dispersed medium and dispersion phase interact with each other:
- Lyophilic: Colloids in which the dispersed phase particles have great affinity for the dispersion medium particles are also known as lyophilic or water-loving colloids.
- Lyophobic: Whenever there is no affinity between the particles of the two interacting phases/mediums, the solution is also called lyophilic, or water-hating.
Here is a table comparing the two:
|
Property |
Lyophillic |
Lyophobic |
|
Stability |
More stable |
Less stable |
|
Surface tension |
Lower than the medium’s |
Same as the medium’s |
|
Reversibility |
Reversible |
Irreversible |
|
Viscosity |
Higher than the medium’s |
Same as the medium’s |
|
Migration |
Particles don’t carry charge and so don’t migrate in an electric field |
Particles migrate to either of the electrodes in an electric field as they carry charge |
|
Addition of electrolyte |
Small quantities of electrolyte have little to no effect |
Causes coagulation immediately |
|
Hydration |
Extensive hydration takes place |
No hydration |
|
EXAMPLES |
Starch, gelatin, gum |
Metal sulphides, gold, silver |
Classification based only on dispersed phase
We can also classify colloids based solely on the dispersed phase:
- Multimolecular colloids formed by an aggregation of a large number of molecules.
- Macromolecular colloids having molecules of large size.
- Associated colloids formed by an aggregation of ions in a concentrated solution.
Mechanical properties of colloids:
- Brownian motion: When you observe a colloid under a microscope, you notice particles moving rapidly in a zigzag motion. This is also called Brownian motion, a result of the constant collision between the particles of the dispersion medium and the dispersed phase.
- Diffusion: Colloid particles diffuse from an area of higher concentration to an area of lower concentration
- Sedimentation: Colloids take an extremely long time to form sediment
Coagulation:
There are a few ways that colloids end up coagulating. Here are a few ways to make it happen:
- Electrophoresis: Colloidal particles move towards electrodes, and subsequently precipitate after being in contact for a long time.
- Mixing two oppositely charged sols: When sols of opposite charges are mixed, they are neutralised, consequently giving rise to precipitation.
- Boiling: Adsorbed layers get disturbed on boiling. This consequently reduces the charge on particles and results in precipitation.
- Persistent dialysis: Persistent dialysis removes almost all traces of electrolytes, rendering colloids unstable.
- Adding electrolytes with opposite charge: This is also in the same vein as point 2. Oppositely charged electrolytes cause neutralisation and coagulation from the ions of the electrolyte.
Application of colloids in real life:
- Purification of water using alum
- Coagulation in the rubber and tanning industries
- Artificial rain using electrified sand
- Blood clotting
- Photographic plates
Colloidal chemistry is also discussed in the majority of the chapter Surface Chemistry in 12th CBSE chemistry. This is an important chapter for board exams, and also competitive entrances like IITJEE, BITSAT, etc. To learn more about colloid preparation, micelles, emulsions and the like, visit edureify.com today!
Master Your Coding Skills with BootSelf AI
If you're looking to enhance your coding abilities and upskill in artificial intelligence, look no further than the BootSelf AI app. This innovative platform provides AI-based coding lessons that are tailored to your individual learning pace.
Available on both iOS and Android, you can download the BootSelf AI app and start mastering coding skills today:





