Science is used to study the world around us. For centuries, chemists have been studying all the states of matter and their properties. Today, we can use the results of these studies to answer several questions. Why do scuba divers get bends? How do syringes work? You can answer these questions by studying Boyle’s law.
GAS LAWS
Many scientists have attempted to summarise the behaviour of gases mathematically. As a result, we have now got 3 major Gas Laws, namely Boyle’s Law, Charle’s Law and also Gay-Lussac’s Law. A 4th law also called, Avogadro’s law, is arrived at by using all of the previously mentioned laws.
These laws apply to an ideal gas, which can be characterised as follows:
- Gas particles are constantly in random motion. These can collide with container walls and also with each other. In between collisions, they only move in straight lines.
- Particles have no volume. Their size is negligible in comparison to the size of the spaces between them.
- There is no attraction or repulsion between the particles.
- Gas pressure is due to the molecules colliding with the walls of the container. No energy is lost or gained from collisions.
- The time a collision takes is negligible in comparison to the time between successive collisions.
- The kinetic energy of a gas is a measure of its Kelvin temperature. Individual gas molecules have different speeds, but the temperature and kinetic energy of the gas refer to the average of these speeds.
- The average kinetic energy of a gas particle is directly proportional to the temperature.
- All gases at a given temperature also have the same average kinetic energy.
- Lighter gas molecules move faster than heavier molecules.
BOYLE’S LAW
Boyle’s law states that
“the absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies, if the temperature and amount of gas remain unchanged within a closed system”
This statement has several elements:
- It applies only to ideal gases
- Applicable to closed systems only
- It also requires the temperature and amount of gas not to change
Keeping the above conditions in mind, let us take the pressures p1 and p2 of a gas at two different times t1 and t2, where the ideal gas correspondingly occupies volumes v1 and v2.
According to Boyle’s law,
p1 1/v1
p2 1/v2
Therefore, we get
p1 =k v1
p2 =kv2
Thus, we get p1/p2 = v2/v1, which is the mathematical form of Boyle’s law.
EXPLANATION OF BOYLE’S LAW
Going forward from the kinetic theory of gases, we know that the molecules of an ideal gas are constantly colliding inelastic collisions. Just look at this picture of the same jar when the volume is changed by using a piston, assuming that no gas escapes and the temperature remains the same:
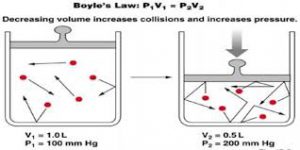
Decreasing the volume will also increase the number of collisions of the gas molecules. This, in turn, creates more collisions of the molecules with the piston, and thus increases the pressure on the piston. One observes this as an increase in the pressure of the gas.
GRAPH
Here is a graph demonstrating Boyle’s law:
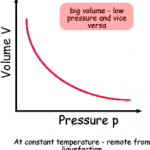
Notice that the pressure decreases as the volume increases.
Look at the same curve at different temperatures:
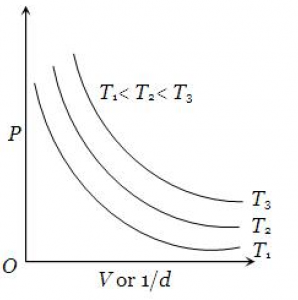
Boyle’s law is one of the most important laws in Physics and Chemistry. This appears in the Kinetic Theory of Gases chapter in physics and also in the Gaseous state chapter in chemistry. Boyle’s law is a part of many numericals in both board exams as well as entrances and other competitive science exams.
To learn more about gas laws and numericals, visit www.edureify.com today!
Master Your Coding Skills with BootSelf AI
If you're looking to enhance your coding abilities and upskill in artificial intelligence, look no further than the BootSelf AI app. This innovative platform provides AI-based coding lessons that are tailored to your individual learning pace.
Available on both iOS and Android, you can download the BootSelf AI app and start mastering coding skills today:




