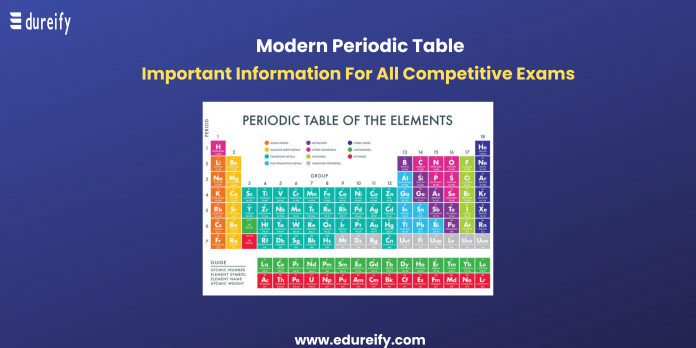
The modern periodic table is one of the most important parts of Chemistry on which the entire understanding is based. The periodic table is one of the first things students need to grasp to have an easy understanding of the rest of the concepts in Chemistry. Edureify brings to all a detailed article on the periodic table to help students grasp the concept.
Read on to know in detail about the Periodic Table.
What is the Periodic Table?
To go by the bookish definition,
“Periodic table is the organized array of all the chemical elements in order of increasing atomic number, i.e., the total number of protons in the atomic nucleus.”
Some important and quick points regarding the Modern Periodic Table
Here are some important facts regarding the Modern Periodic Table’s founder-
| Points | Particulars |
| Who invented the modern periodic table? | Henry Moseley |
| When was the invention made? | 1913 |
| Who was first able to arrange all the known elements? | Dmitri Mendeleev- gave the Mendeleev’s periodic law |
| Who grouped the elements into triads? | Dobereiner |
| Who gave the law of octaves? | Newlands |
Mendeleev Periodic Table
Dmitri Mendeleev, the father of the periodic table, had first put forth the known elements and arranged them in a tabular format. Mendeleev began his journey of arranging the periodic table in 1869.
The vital difference between Mendeleev’s periodic table and the modern periodic table that is an important part of all the competitive exams is-
“Mendeleev had designed his periodic table based on increasing atomic mass, while the modern periodic law is based on the increasing order of the atomic numbers.”
What is the modern periodic law?
According to the modern periodic law, the physical and chemical properties of the elements are the periodic functions of their atomic numbers.
Some of the features of the modern periodic law are-
- The periodic table is arranged in a manner where a row is a period and a column is a group
- The elements are grouped from left to right and top to bottom in their increasing atomic number order
- The elements in the same group have the same valence electron configuration and similar chemical properties
- On the other hand, elements in the same period have an increasing number of valence electrons where the number of energy sub-levels per energy level increases as the energy level of the atom increases
List of first 50 elements of the periodic table
| S.no | Symbol | Name |
| 1. | H | Hydrogen |
| 2. | He | Helium |
| 3. | Li | Lithium |
| 4. | Be | Beryllium |
| 5. | B | Boron |
| 6. | C | Carbon |
| 7. | N | Nitrogen |
| 8. | O | Oxygen |
| 9. | F | Fluorine |
| 10. | Ne | Neon |
| 11. | Na | Sodium |
| 12. | Mg | Magnesium |
| 13. | Al | Aluminum |
| 14. | Si | Silicon |
| 15. | P | Phosphorus |
| 16. | S | Sulphur |
| 17. | Cl | Chlorine |
| 18. | Ar | Argon |
| 19. | K | Potassium |
| 20. | Ca | Calcium |
| 21. | Sc | Scandium |
| 22. | Ti | Titanium |
| 23. | V | Vanadium |
| 24. | Cr | Chromium |
| 25. | Mn | Manganese |
| 26. | Fe | Iron |
| 27. | Co | Cobalt |
| 28. | Ni | Nickel |
| 29. | Cu | Copper |
| 30. | Zn | Zinc |
| 31. | Ga | Gallium |
| 32. | Ge | Germanium |
| 33. | As | Arsenic |
| 34. | Se | Selenium |
| 35. | Br | Bromine |
| 36. | Kr | Krypton |
| 37. | Rb | Rubidium |
| 38. | Sr | Strontium |
| 39. | Y | Yttrium |
| 40. | Zr | Zirconium |
| 41. | Nb | Niobium |
| 42. | Mo | Molybdenum |
| 43. | Tc | Technetium |
| 44. | Ru | Ruthenium |
| 45. | Rh | Rhodium |
| 46. | Pd | Palladium |
| 47. | Ag | Silver |
| 48. | Cd | Cadmium |
| 49. | In | Indium |
| 50. | Sn | Tin |
The periodic table is a vital part of not just understanding Chemistry, but the concept is often a part of many competitive government exams in India. Edureify, therefore, has allotted a particular section for Chemistry where candidates of various competitive exams like NEET and JEE, and students of classes 8-10 of CBSE board to help them better understand and grasp the concepts of Chemistry.
Check out the Edureify website and also download the Edureify AI Learning App to learn more about the Periodic Table and other important concepts of Chemistry. Students and aspirants can also take numerous mock tests, and practice quizzes, and get hold of tons of flashcards and study materials for better preparation for their Chemistry exam with Edureify.
Some FAQs regarding the Modern Periodic Table-
1. Who invented the Modern Periodic Table and when?
Henry Moseley invented the modern periodic table in 1913.
2. What is the Mendeleev periodic law?
Unlike the Modern Periodic Law, the Mendeleev Periodic Law is based on increasing atomic masses.
3. How many elements are there in total in the periodic table?
There are a total of 118 elements in the periodic table.
4. What is the symbol of Titanium?
The symbol of Titanium is Ti.
5. Where can I get all the materials regarding the periodic table for exam preparation?
Edureify! Students will get all the important study materials regarding the periodic table and other important chemistry notes on Edureify.
Master Your Coding Skills with BootSelf AI
If you're looking to enhance your coding abilities and upskill in artificial intelligence, look no further than the BootSelf AI app. This innovative platform provides AI-based coding lessons that are tailored to your individual learning pace.
Available on both iOS and Android, you can download the BootSelf AI app and start mastering coding skills today: